The luminal AAA+ ATPase torsinA mediates distinct mechanisms of nuclear-cytoplasmic communication by adopting different functional assembly states
4.5 (688) · € 24.50 · In Magazzino

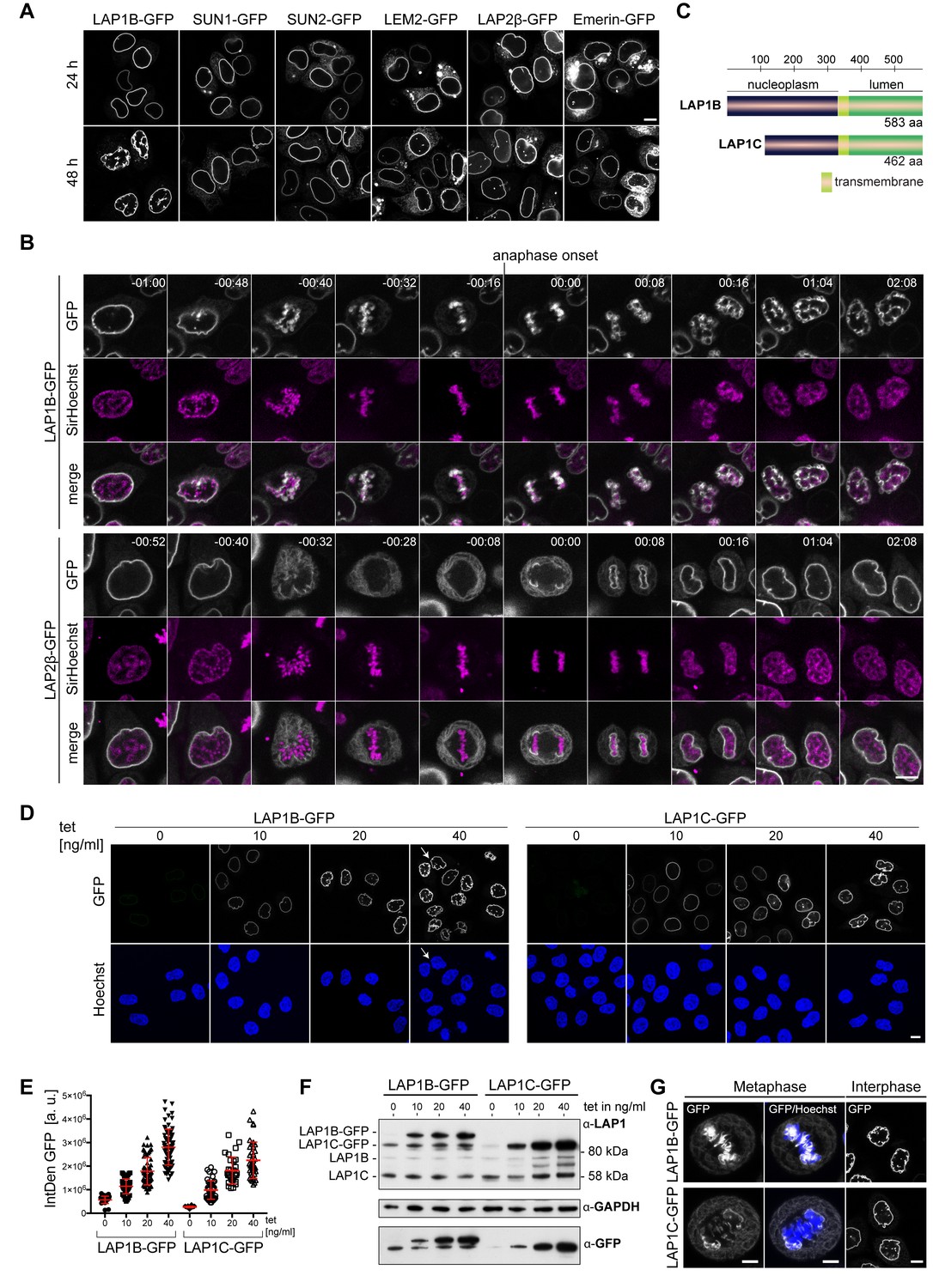
Torsin ATPases influence chromatin interaction of the Torsin regulator LAP1

Figure 2 from The LINC and NPC relationship – it's complicated!

The assembly of stable TAN lines requires both TA and LAP1. (A)

Cells, Free Full-Text
Transport and Communication Across the Nuclear Envelope

Dynamic functional assembly of the Torsin AAA+ ATPase and its modulation by LAP1

Transport and Communication Across the Nuclear Envelope

PDF) The luminal AAA+ ATPase torsinA mediates distinct mechanisms of nuclear -cytoplasmic communication by adopting different functional assembly states

LINC Complexes Form by Binding of Three KASH Peptides to Domain Interfaces of Trimeric SUN Proteins: Cell
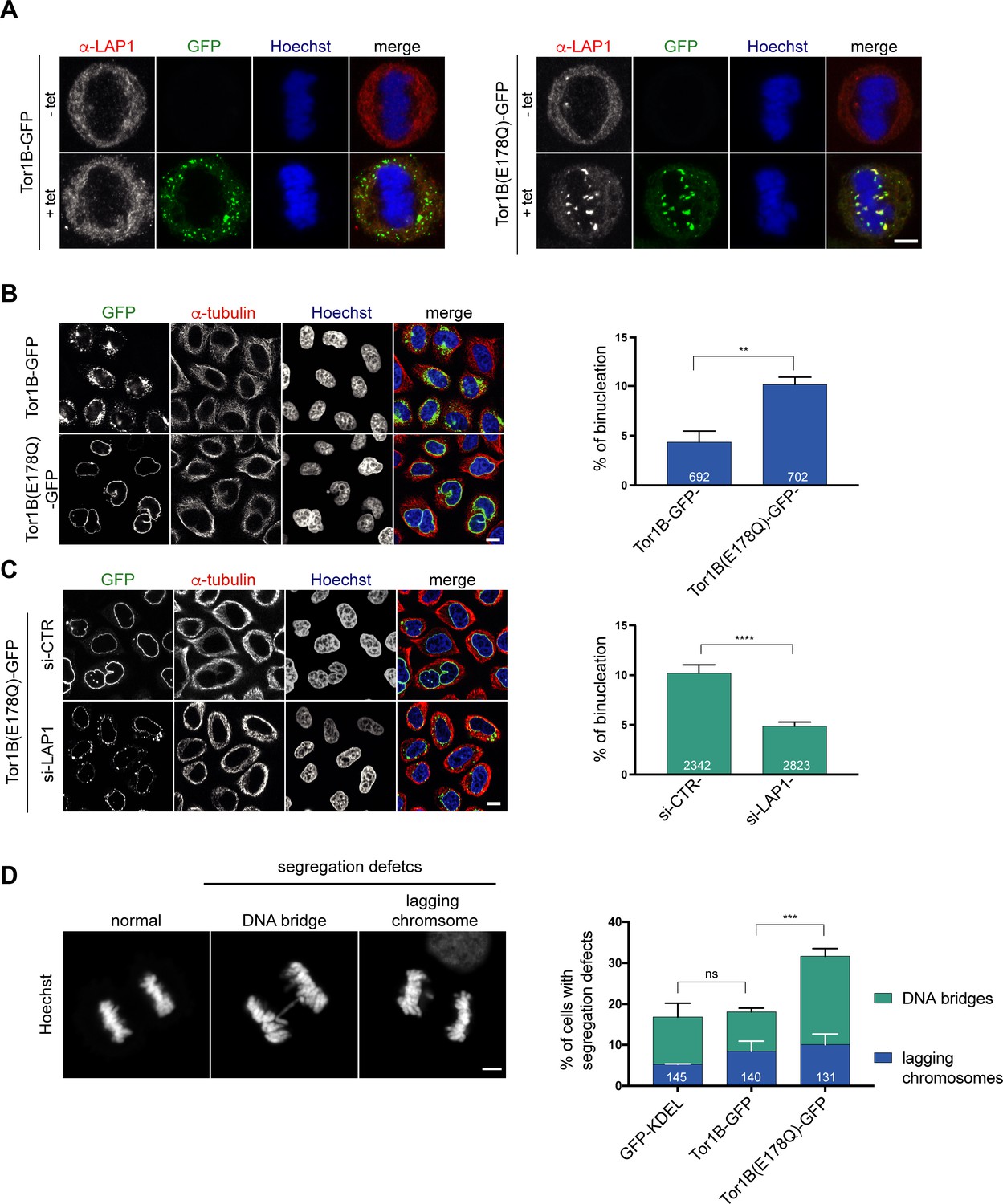
Torsin ATPases influence chromatin interaction of the Torsin regulator LAP1

Aberrant Cellular Behavior of Mutant TorsinA Implicates Nuclear Envelope Dysfunction in DYT1 Dystonia

LAP1 and LULL1 luminal domains are predicted to adopt an AAA+-like
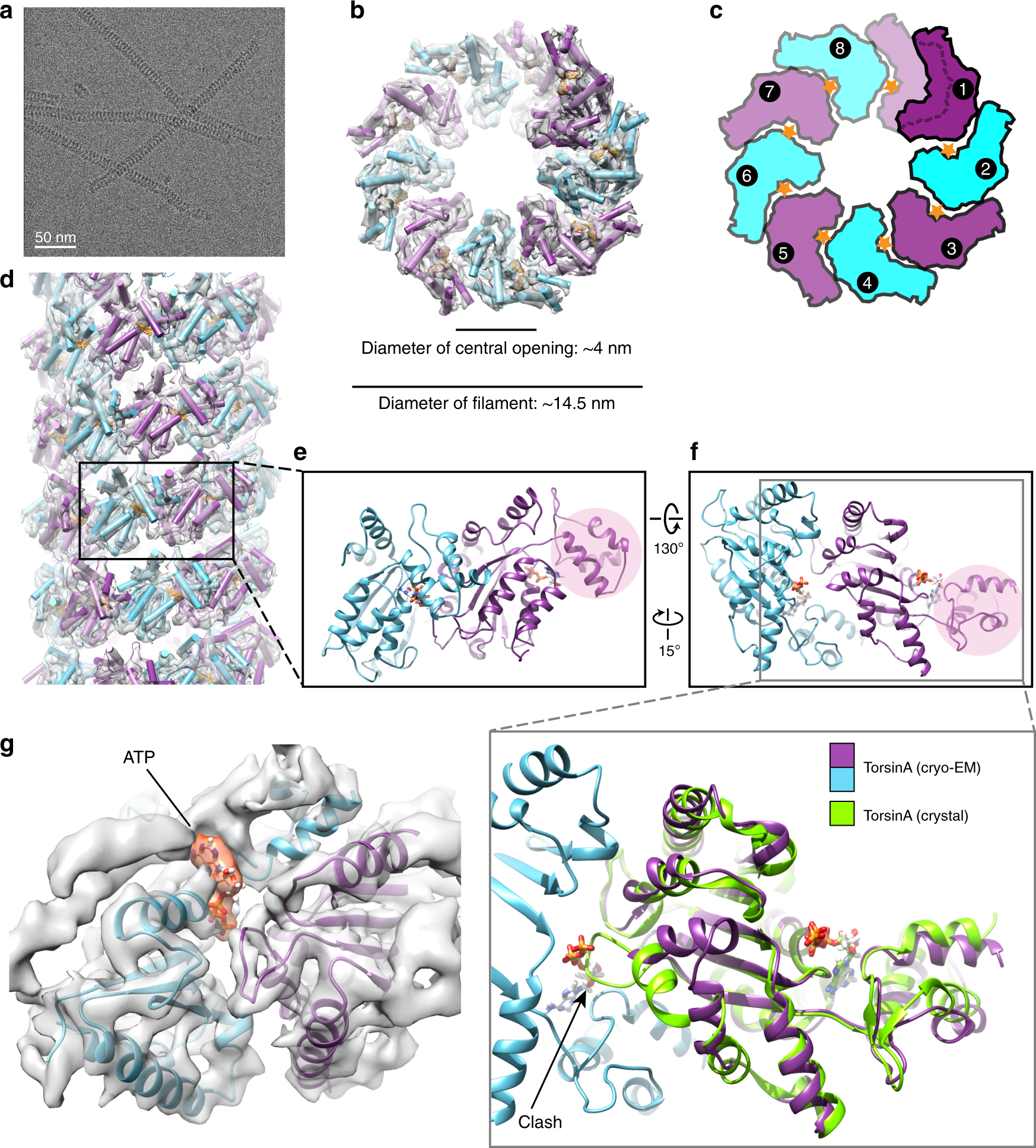
The AAA + ATPase TorsinA polymerizes into hollow helical tubes with 8.5 subunits per turn







